1: Spatial and Measurement Functions
Demonstrate the usage of spatial measurement functions.
[1]:
import matplotlib.pyplot as plt
import seaborn as sns
import anndata as ad
import spatialcells as spc
In this tutorial, we will be demonstrating the spatial measurements functions by studying the distribution of MITF+ cells among SOX10+ tumor cells
read and process data
You can download the data from here. Please refer to the original paper for the details.
[2]:
adata = ad.read_h5ad("../../data/MEL1_adata.h5ad")
[3]:
spc.prep.setGate(adata, "SOX10_cellRingMask", 7.9, debug=True)
spc.prep.setGate(adata, "MITF_cellRingMask", 6.3, debug=True)
spc.prep.setGate(adata, "KERATIN_cellRingMask", 6.4, debug=True)
SOX10_cellRingMask_positive
False 566576
True 544009
Name: count, dtype: int64
MITF_cellRingMask_positive
False 851822
True 258763
Name: count, dtype: int64
KERATIN_cellRingMask_positive
False 1067400
True 43185
Name: count, dtype: int64
[4]:
def combine_columns(row):
if row["MITF_cellRingMask_positive"] and row["SOX10_cellRingMask_positive"]:
return "SOX10+MITF+"
elif row["SOX10_cellRingMask_positive"]:
return "SOX10+MITF-"
else:
return "SOX10-"
# Applying the function to create the new phenotype column
adata.obs["pheno"] = adata.obs.apply(combine_columns, axis=1)
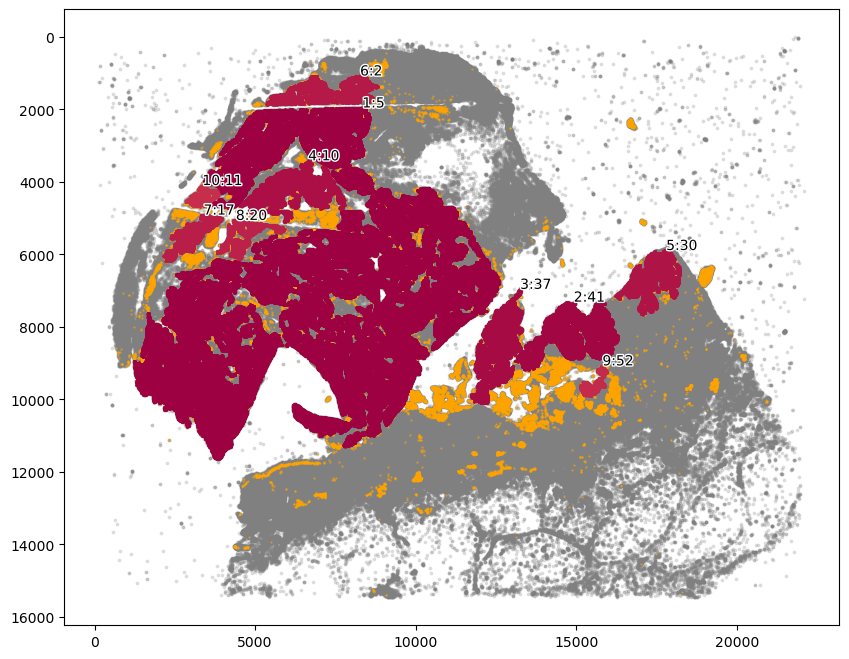
The following steps compute the communities based on SOX10+ cells.
[5]:
markers_of_interest = ["SOX10_cellRingMask_positive"]
communitycolumn = "COI_community"
ret = spc.spatial.getCommunities(
adata, markers_of_interest, eps=50, newcolumn=communitycolumn
)
# Plotting the communities
plot_first_n_clusters = 10
fig, ax = plt.subplots(figsize=(10, 8))
spc.plt.plotCommunities(
adata,
ret,
communitycolumn,
plot_first_n_clusters=plot_first_n_clusters,
s=1,
fontsize=10,
ax=ax,
)
ax.invert_yaxis()
plt.show()
[6]:
# Here we chose the main tumor area.
communityIndexList = [5, 2, 10, 11, 17, 20]
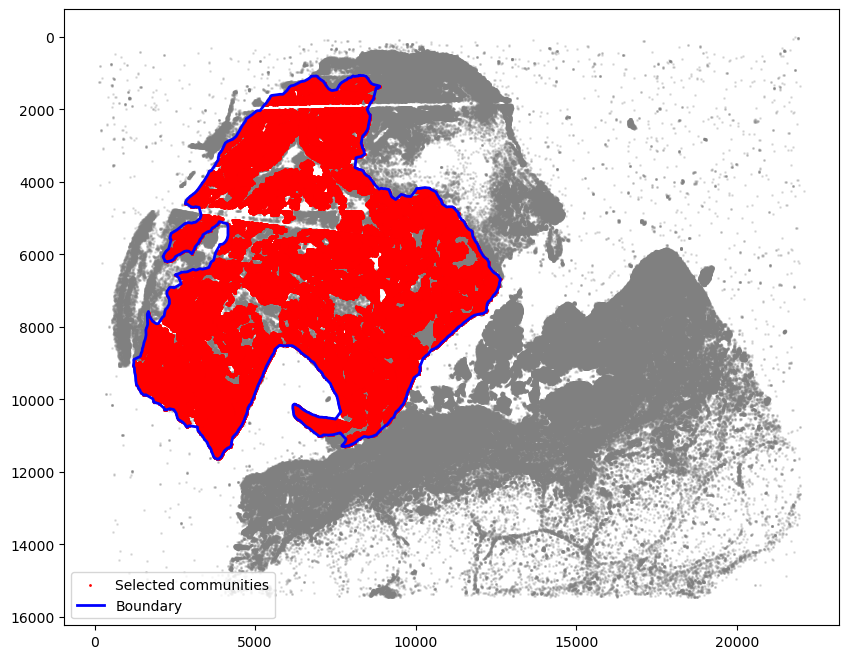
getBoundary
[7]:
boundary = spc.spa.getBoundary(adata, communitycolumn, communityIndexList, alpha=150)
pruned_boundary = spc.spa.pruneSmallComponents(
boundary, min_edges=20, holes_min_edges=200
)
[8]:
markersize = 1
fig, ax = plt.subplots(figsize=(10, 8))
## all points
ax.scatter(
*zip(*adata.obs[["X_centroid", "Y_centroid"]].to_numpy()),
s=markersize,
color="grey",
alpha=0.2
)
# Points in selected commnities
xy = adata.obs[adata.obs[communitycolumn].isin(communityIndexList)][
["X_centroid", "Y_centroid"]
].to_numpy()
ax.scatter(xy[:, 0], xy[:, 1], s=markersize, color="r", label="Selected communities")
# Bounds of points in selected commnities
spc.plt.plotBoundary(pruned_boundary, ax=ax, linewidth=2, color="b", label="Boundary")
ax.invert_yaxis()
ax.legend(loc="lower left")
plt.show()
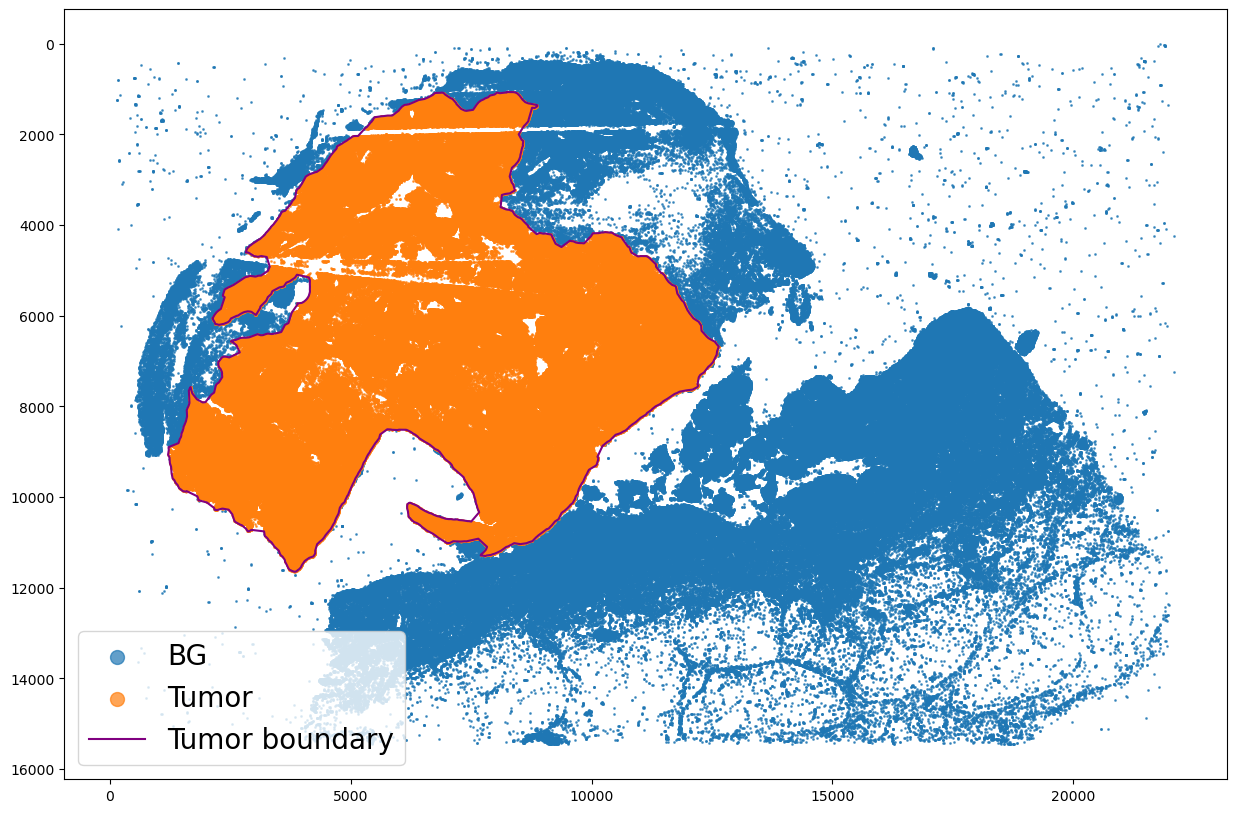
assignPointsToRegion
[9]:
regions = ["Tumor"]
boundaries_list = [pruned_boundary]
spc.spatial.assignPointsToRegions(
adata, boundaries_list, regions, assigncolumn="region", default="BG"
)
719117it [00:58, 12263.00it/s]
Assigned points to region: Tumor
[10]:
point_size = 1
fig, ax = plt.subplots(figsize=(15, 10))
for region in sorted(set(adata.obs["region"])):
tmp = adata[adata.obs.region == region]
ax.scatter(
*zip(*tmp.obs[["X_centroid", "Y_centroid"]].to_numpy()),
s=point_size,
alpha=0.7,
label=region
)
# Bounds of points in selected commnities
spc.plt.plotBoundary(pruned_boundary, color="purple", label="Tumor boundary")
ax.invert_yaxis()
plt.legend(loc="lower left", markerscale=10, fontsize=20)
plt.show()
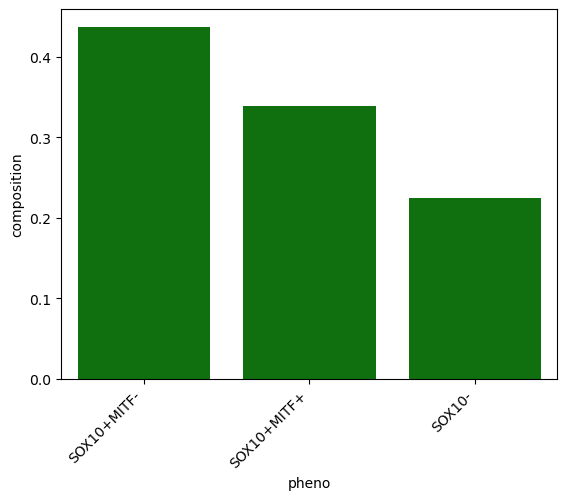
First we calculate the overall Tumor region composition.
[11]:
roi_comp = spc.msmt.getRegionComposition(adata, "pheno", regions=["Tumor"])
roi_comp
[11]:
| pheno | cell_count | composition | |
|---|---|---|---|
| 0 | SOX10+MITF- | 252594 | 0.437427 |
| 1 | SOX10+MITF+ | 195279 | 0.338172 |
| 2 | SOX10- | 129581 | 0.224401 |
[12]:
sns.barplot(x="pheno", y="composition", color="green", data=roi_comp)
plt.xticks(rotation=45, ha="right")
plt.show()
Tumor area and density
[13]:
area = spc.msmt.getRegionArea(pruned_boundary)
print("area: ", area)
density = spc.msmt.getRegionDensity(
adata,
pruned_boundary,
region_subset=["Tumor"])
print("All cell density/100px2: ", density.values[0] * 10000, "\n")
sox10_density = spc.msmt.getRegionDensity(
adata,
pruned_boundary,
region_subset=["Tumor"],
phenotype_col=["SOX10_cellRingMask_positive"],
)
print("SOX10 positive cell density in tumor/100px2:\n", sox10_density * 10000, "\n")
pheno_density = spc.msmt.getRegionDensity(
adata,
pruned_boundary,
region_subset=["Tumor"],
phenotype_col=["pheno"]
)
print("pheno density in tumor/100px2:\n", pheno_density * 10000)
area: 66103699.31245056
All cell density/100px2: 87.35577675775208
SOX10 positive cell density in tumor/100px2:
SOX10_cellRingMask_positive
True 67.753092
False 19.602685
Name: count, dtype: float64
pheno density in tumor/100px2:
pheno
SOX10+MITF- 38.211780
SOX10+MITF+ 29.541312
SOX10- 19.602685
Name: count, dtype: float64
Tumor organization
[14]:
centroid = spc.msmt.getRegionCentroid(pruned_boundary)
edge_pt = [2000, 2000]
[15]:
help(spc.msmt.getDistanceFromPoint)
Help on function getDistanceFromPoint in module spatialcells.measurements._getDistanceFromPoint:
getDistanceFromPoint(adata, point, x='X_centroid', y='Y_centroid', region_col='region', region_subset=None, metric='angular', name='distance', inplace=True, binned=False, binsize=10)
Get the distance of each cell from a point.
:param adata: Anndata object
:param point: iterable coordinate of a point in (x, y) to calculate distance from
:param x: Name of the column containing the x coordinate. Default is "X_centroid".
:param y: Name of the column containing the y coordinate. Default is "Y_centroid".
:param region_col: Name of the column containing the region. Default is "region".
:param region_subset: List of regions to consider. If None, consider all cells.
:param metric: metric to use for distance calculation.
Metric can be "angular" or "euclidean". Default is "angular".
:param name: Name of the column to store the distance in. Default is "distance".
:param inplace: If True, add the distance column to adata.obs. If False, return a copy
:param binned: If True, bin the distances into bins of size binsize.
:param binsize: Size of the bins to use for binning. Default is 10.
:returns: If inplace is False, return a copy of adata with the distance column added
[16]:
spc.msmt.getDistanceFromPoint(
adata,
centroid,
region_subset=["Tumor"],
metric="euclidean",
name="euc_distance",
binned=True,
binsize=1000,
)
spc.msmt.getDistanceFromPoint(
adata,
centroid,
region_subset=["Tumor"],
metric="angular",
name="ang_distance",
binned=True,
binsize=45,
)
spc.msmt.getDistanceFromPoint(
adata,
edge_pt,
region_subset=["Tumor"],
metric="euclidean",
name="euc_edge",
binned=True,
binsize=1500,
)
spc.msmt.getDistanceFromPoint(
adata,
edge_pt,
region_subset=["Tumor"],
metric="angular",
name="ang_edge",
binned=True,
binsize=30,
)
[17]:
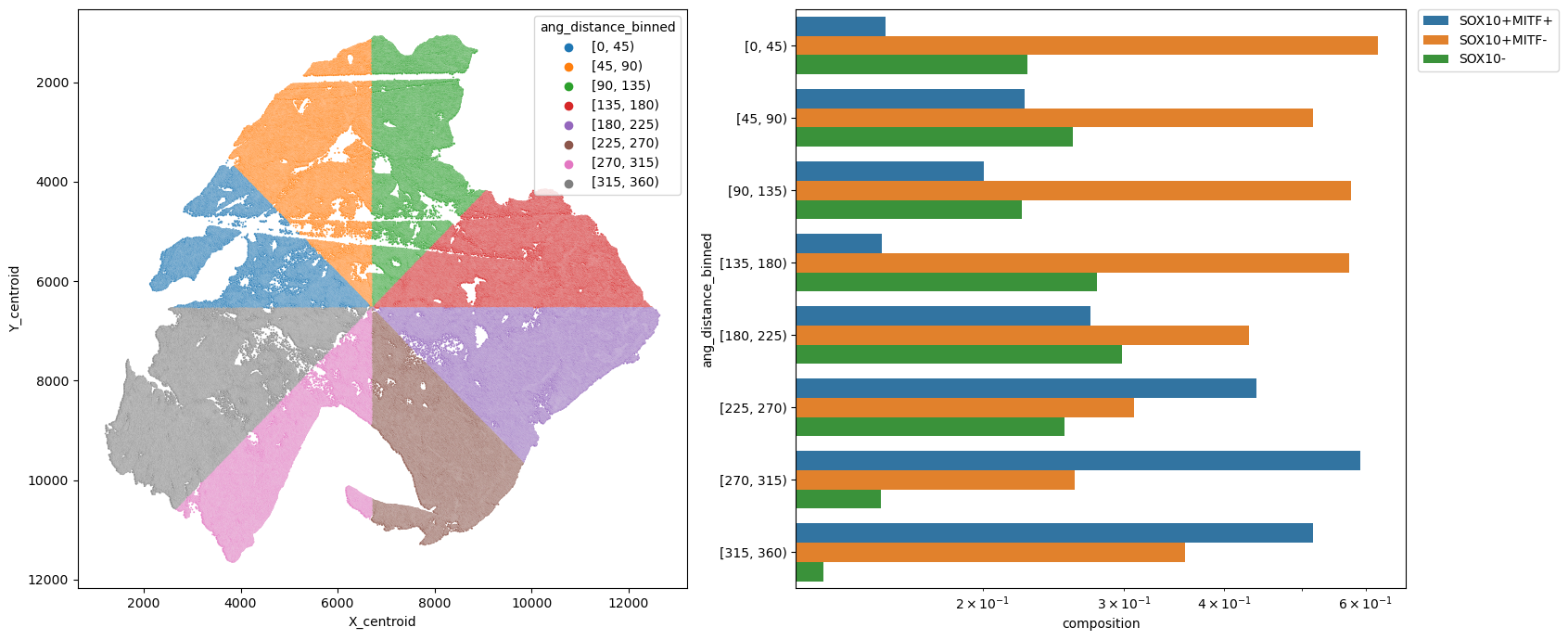
metric_col = "ang_distance_binned"
pheno = "pheno"
fig, [ax1, ax2] = plt.subplots(1, 2, figsize=(17, 7))
sns.scatterplot(
data=adata.obs,
x="X_centroid",
y="Y_centroid",
hue=metric_col,
alpha=0.8,
s=2,
ax=ax1
)
ax1.invert_yaxis()
ax1.legend(loc="upper right", title=metric_col)
df = spc.msmt.getRegionComposition(
adata, [metric_col, pheno], regions=["Tumor"]
).sort_values(by=metric_col, ascending=False)
sns.barplot(data=df, y=metric_col, x="composition", hue=pheno, log=True, ax=ax2)
ax2.legend(bbox_to_anchor=(1.02, 1), loc="upper left", borderaxespad=0)
plt.tight_layout()
plt.show()
/Users/ghwan/opt/anaconda3/envs/spatialcells/lib/python3.10/site-packages/seaborn/categorical.py:253: FutureWarning: The default of observed=False is deprecated and will be changed to True in a future version of pandas. Pass observed=False to retain current behavior or observed=True to adopt the future default and silence this warning.
grouped_vals = vals.groupby(grouper)
/Users/ghwan/opt/anaconda3/envs/spatialcells/lib/python3.10/site-packages/seaborn/categorical.py:253: FutureWarning: The default of observed=False is deprecated and will be changed to True in a future version of pandas. Pass observed=False to retain current behavior or observed=True to adopt the future default and silence this warning.
grouped_vals = vals.groupby(grouper)
[18]:
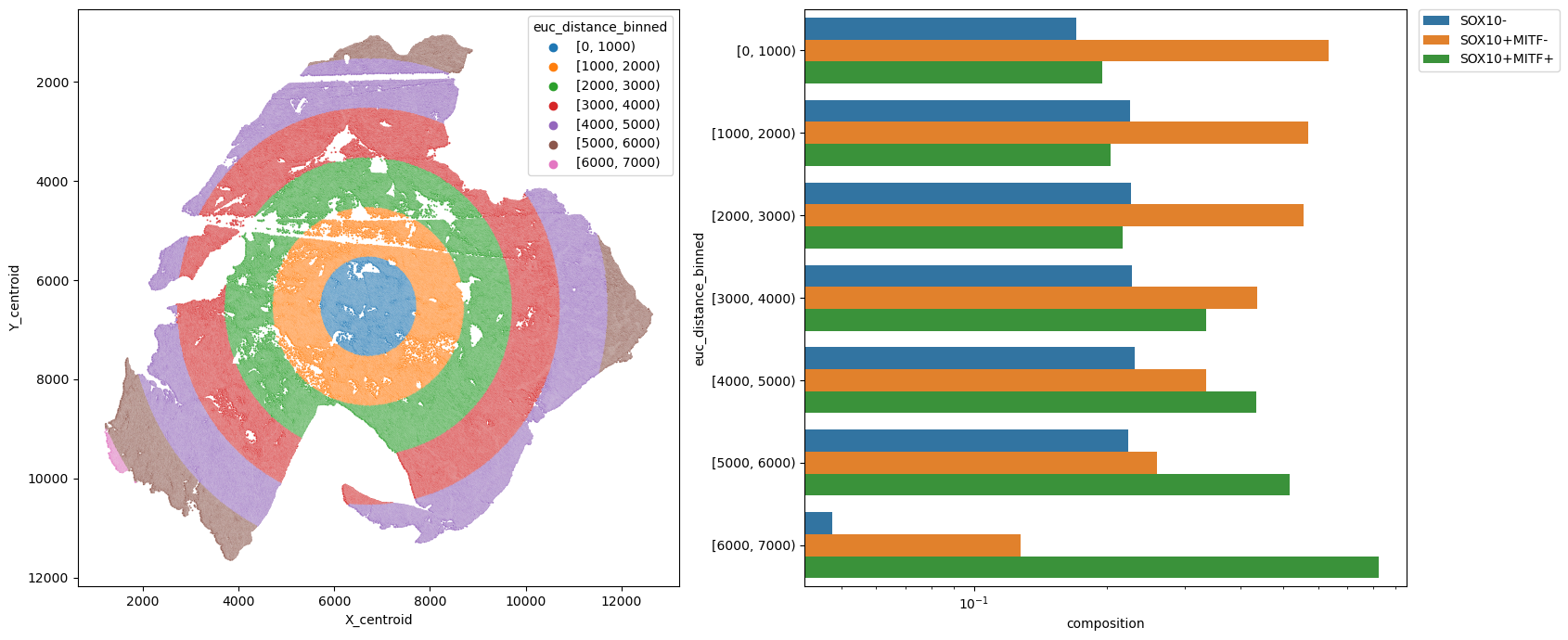
metric_col = "euc_distance_binned"
pheno = "pheno"
df = spc.msmt.getRegionComposition(
adata, [metric_col, pheno], regions=["Tumor"]
).sort_values(by=metric_col, ascending=False)
fig, [ax1, ax2] = plt.subplots(1, 2, figsize=(17, 7))
sns.scatterplot(
data=adata.obs,
x="X_centroid",
y="Y_centroid",
hue=metric_col,
alpha=0.8,
s=2,
ax=ax1,
)
ax1.invert_yaxis()
ax1.legend(loc="upper right", title=metric_col)
sns.barplot(data=df, y=metric_col, x="composition", hue=pheno, log=True, ax=ax2)
ax2.legend(bbox_to_anchor=(1.02, 1), loc="upper left", borderaxespad=0)
plt.tight_layout()
plt.show()
/Users/ghwan/opt/anaconda3/envs/spatialcells/lib/python3.10/site-packages/seaborn/categorical.py:253: FutureWarning: The default of observed=False is deprecated and will be changed to True in a future version of pandas. Pass observed=False to retain current behavior or observed=True to adopt the future default and silence this warning.
grouped_vals = vals.groupby(grouper)
/Users/ghwan/opt/anaconda3/envs/spatialcells/lib/python3.10/site-packages/seaborn/categorical.py:253: FutureWarning: The default of observed=False is deprecated and will be changed to True in a future version of pandas. Pass observed=False to retain current behavior or observed=True to adopt the future default and silence this warning.
grouped_vals = vals.groupby(grouper)
[19]:
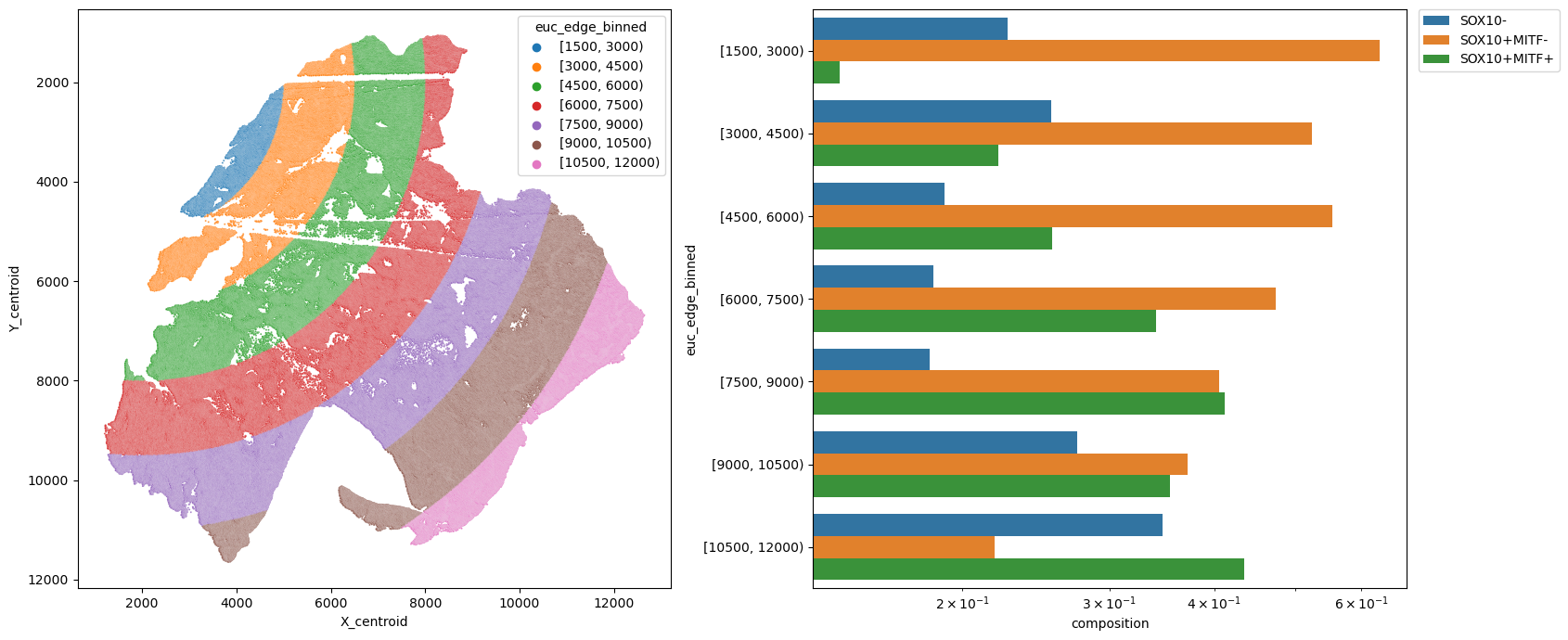
metric_col = "euc_edge_binned"
pheno = "pheno"
fig, [ax1, ax2] = plt.subplots(1, 2, figsize=(17, 7))
sns.scatterplot(
data=adata.obs,
x="X_centroid",
y="Y_centroid",
hue=metric_col,
alpha=0.8,
s=2,
ax=ax1,
)
ax1.invert_yaxis()
ax1.legend(loc="upper right", title=metric_col)
df = spc.msmt.getRegionComposition(
adata, [metric_col, pheno], regions=["Tumor"]
).sort_values(by=metric_col, ascending=False)
sns.barplot(data=df, y=metric_col, x="composition", hue=pheno, log=True, ax=ax2)
ax2.legend(bbox_to_anchor=(1.02, 1), loc="upper left", borderaxespad=0)
plt.tight_layout()
plt.show()
/Users/ghwan/opt/anaconda3/envs/spatialcells/lib/python3.10/site-packages/seaborn/categorical.py:253: FutureWarning: The default of observed=False is deprecated and will be changed to True in a future version of pandas. Pass observed=False to retain current behavior or observed=True to adopt the future default and silence this warning.
grouped_vals = vals.groupby(grouper)
/Users/ghwan/opt/anaconda3/envs/spatialcells/lib/python3.10/site-packages/seaborn/categorical.py:253: FutureWarning: The default of observed=False is deprecated and will be changed to True in a future version of pandas. Pass observed=False to retain current behavior or observed=True to adopt the future default and silence this warning.
grouped_vals = vals.groupby(grouper)
[20]:
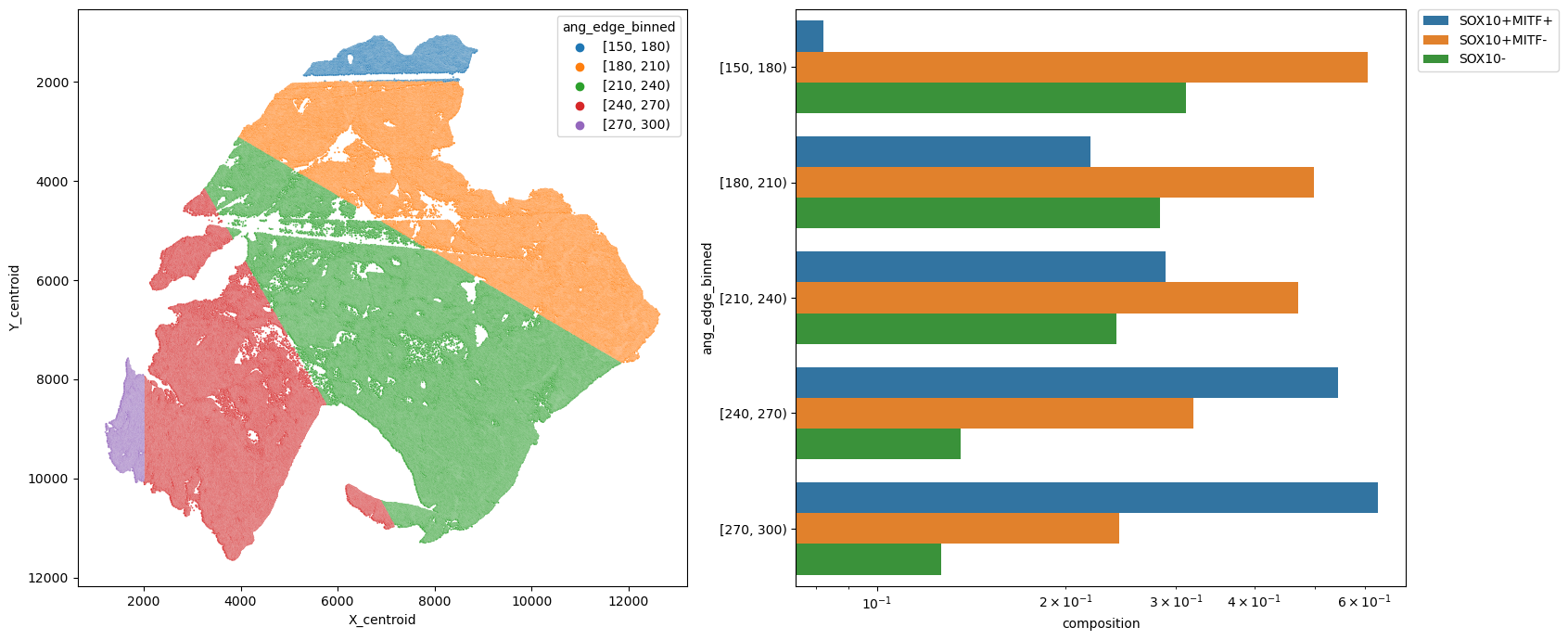
metric_col = "ang_edge_binned"
pheno = "pheno"
fig, [ax1, ax2] = plt.subplots(1, 2, figsize=(17, 7))
sns.scatterplot(
data=adata.obs,
x="X_centroid",
y="Y_centroid",
hue=metric_col,
alpha=0.8,
s=2,
ax=ax1,
)
ax1.invert_yaxis()
ax1.legend(loc="upper right", title=metric_col)
df = spc.msmt.getRegionComposition(
adata, [metric_col, pheno], regions=["Tumor"]
).sort_values(by=metric_col, ascending=False)
sns.barplot(data=df, y=metric_col, x="composition", hue=pheno, log=True, ax=ax2)
ax2.legend(bbox_to_anchor=(1.02, 1), loc="upper left", borderaxespad=0)
plt.tight_layout()
plt.show()
/Users/ghwan/opt/anaconda3/envs/spatialcells/lib/python3.10/site-packages/seaborn/categorical.py:253: FutureWarning: The default of observed=False is deprecated and will be changed to True in a future version of pandas. Pass observed=False to retain current behavior or observed=True to adopt the future default and silence this warning.
grouped_vals = vals.groupby(grouper)
/Users/ghwan/opt/anaconda3/envs/spatialcells/lib/python3.10/site-packages/seaborn/categorical.py:253: FutureWarning: The default of observed=False is deprecated and will be changed to True in a future version of pandas. Pass observed=False to retain current behavior or observed=True to adopt the future default and silence this warning.
grouped_vals = vals.groupby(grouper)
Get distance from the epidermis layer
[21]:
# find skin cell communities
marker = ["KERATIN_cellRingMask_positive"]
communitycolumn = "epi_community"
ret = spc.spatial.getCommunities(adata, marker, eps=100, newcolumn=communitycolumn)
# Plotting the communities
plot_first_n_clusters = 10
fig, ax = plt.subplots(figsize=(10, 8))
spc.plt.plotCommunities(
adata,
ret,
communitycolumn,
plot_first_n_clusters=plot_first_n_clusters,
s=0.1,
fontsize=10,
ax=ax,
)
ax.invert_yaxis()
plt.show()
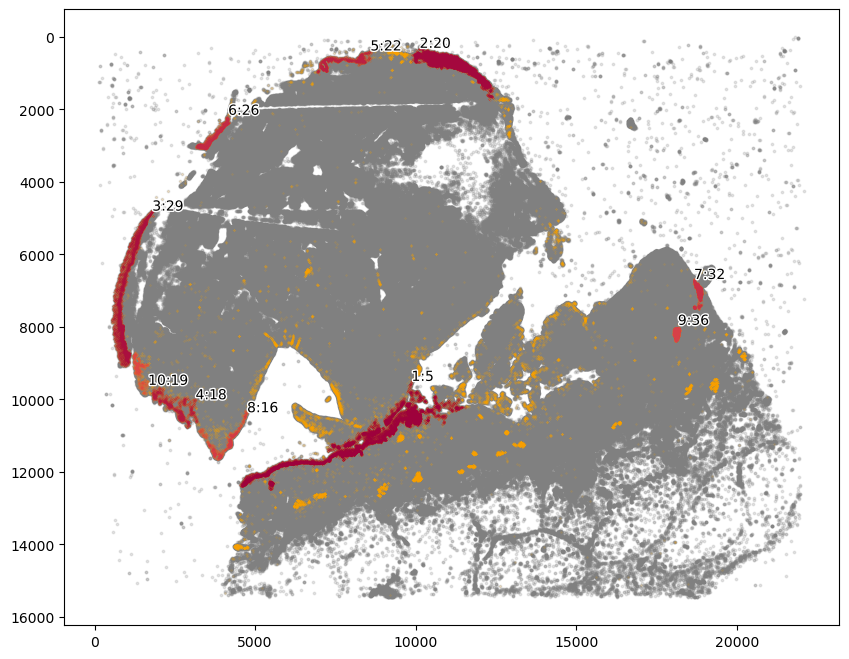
[22]:
communityIndexList = [22, 26, 29]
epi_boundary = spc.spa.getBoundary(
adata, communitycolumn, communityIndexList, alpha=3000
)
markersize = 1
fig, ax = plt.subplots(figsize=(6, 5))
## all points
ax.scatter(
*zip(*adata.obs[["X_centroid", "Y_centroid"]].to_numpy()),
s=markersize,
color="grey",
alpha=0.2
)
# Points in selected commnities
xy = adata.obs[adata.obs[communitycolumn].isin(communityIndexList)][
["X_centroid", "Y_centroid"]
].to_numpy()
ax.scatter(xy[:, 0], xy[:, 1], s=markersize, color="r")
spc.plt.plotBoundary(epi_boundary, ax=ax, linewidth=1.5, color="k")
ax.invert_yaxis()
plt.show()
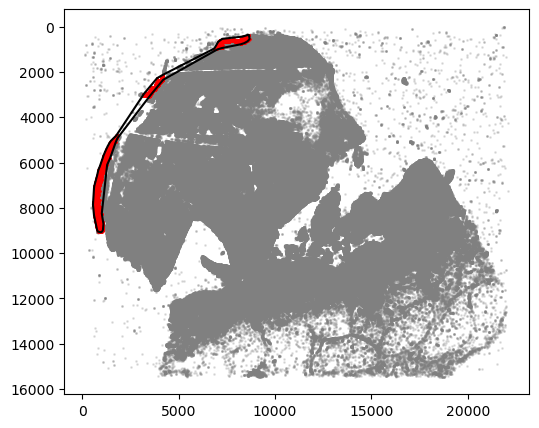
For demonstration, we included a custom, manually drawn line as well. In general, any Shapely object should work
[23]:
from shapely.geometry import LineString
# manually input the line
shapely_obj = LineString([(12000, 2000), (13000, 3000), (13500, 3800)])
[24]:
help(spc.msmt.getDistanceFromObject)
Help on function getDistanceFromObject in module spatialcells.measurements._getDistanceFromObject:
getDistanceFromObject(adata, object, x='X_centroid', y='Y_centroid', region_col='region', region_subset=None, name='distance', inplace=True, binned=False, binsize=10)
Get the minimum euclidean distance between each cell and a shapely object.
:param adata: Anndata object
:param object: Shapely object to measure distance from
:param x: Name of the column containing the x coordinate. Default is "X_centroid".
:param y: Name of the column containing the y coordinate. Default is "Y_centroid".
:param region_col: Name of the column containing the region. Default is "region".
:param region_subset: List of regions to consider. If None, consider all cells.
:param name: Name of the column to store the distance in. Default is "distance".
:param inplace: If True, add the distance column to adata.obs. If False, return a copy
:param binned: If True, bin the distances into bins of size binsize.
:param binsize: Size of the bins to use for binning. Default is 10.
:returns: If inplace is False, return a copy of adata with the distance column added
[25]:
spc.msmt.getDistanceFromObject(
adata,
epi_boundary,
region_col="region",
region_subset=["Tumor"],
name="distance_from_epidermis",
binned=True,
binsize=1000,
)
spc.msmt.getDistanceFromObject(
adata,
shapely_obj,
region_col="region",
region_subset=["Tumor"],
name="distance_from_object",
binned=True,
binsize=1000,
)
577454it [00:15, 36305.82it/s]
577454it [00:12, 47052.12it/s]
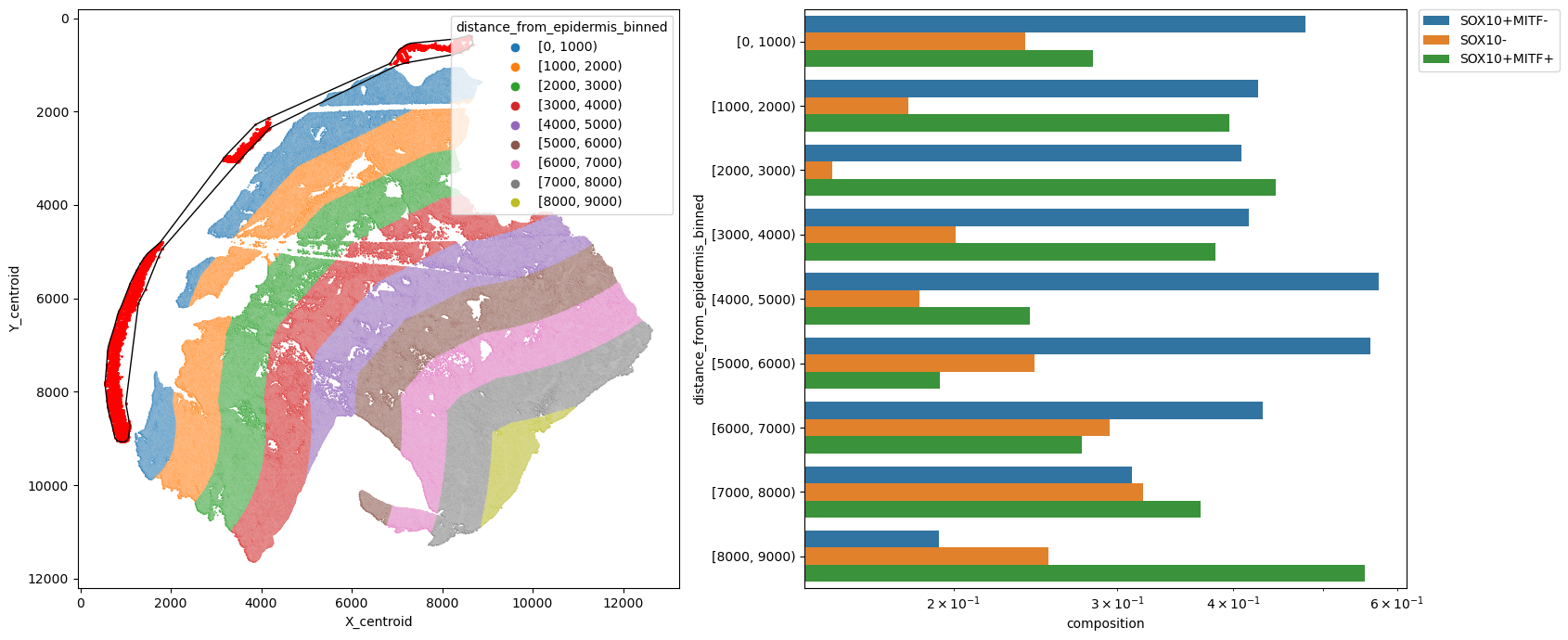
[26]:
metric_col = "distance_from_epidermis_binned"
pheno = "pheno"
fig, [ax1, ax2] = plt.subplots(1, 2, figsize=(17, 7))
# Points in selected commnities
xy = adata.obs[adata.obs[communitycolumn].isin(communityIndexList)][
["X_centroid", "Y_centroid"]
].to_numpy()
ax1.scatter(xy[:, 0], xy[:, 1], s=markersize, color="r")
spc.plt.plotBoundary(epi_boundary, ax=ax1, linewidth=1.0, color="k")
sns.scatterplot(
data=adata.obs,
x="X_centroid",
y="Y_centroid",
hue=metric_col,
alpha=0.8,
s=2,
ax=ax1,
)
ax1.invert_yaxis()
ax1.legend(loc="upper right", title=metric_col)
df = spc.msmt.getRegionComposition(
adata, [metric_col, pheno], regions=["Tumor"]
).sort_values(by=metric_col, ascending=False)
sns.barplot(data=df, y=metric_col, x="composition", hue=pheno, log=True, ax=ax2)
ax2.legend(bbox_to_anchor=(1.02, 1), loc="upper left", borderaxespad=0)
plt.tight_layout()
plt.show()
/Users/ghwan/opt/anaconda3/envs/spatialcells/lib/python3.10/site-packages/seaborn/categorical.py:253: FutureWarning: The default of observed=False is deprecated and will be changed to True in a future version of pandas. Pass observed=False to retain current behavior or observed=True to adopt the future default and silence this warning.
grouped_vals = vals.groupby(grouper)
/Users/ghwan/opt/anaconda3/envs/spatialcells/lib/python3.10/site-packages/seaborn/categorical.py:253: FutureWarning: The default of observed=False is deprecated and will be changed to True in a future version of pandas. Pass observed=False to retain current behavior or observed=True to adopt the future default and silence this warning.
grouped_vals = vals.groupby(grouper)
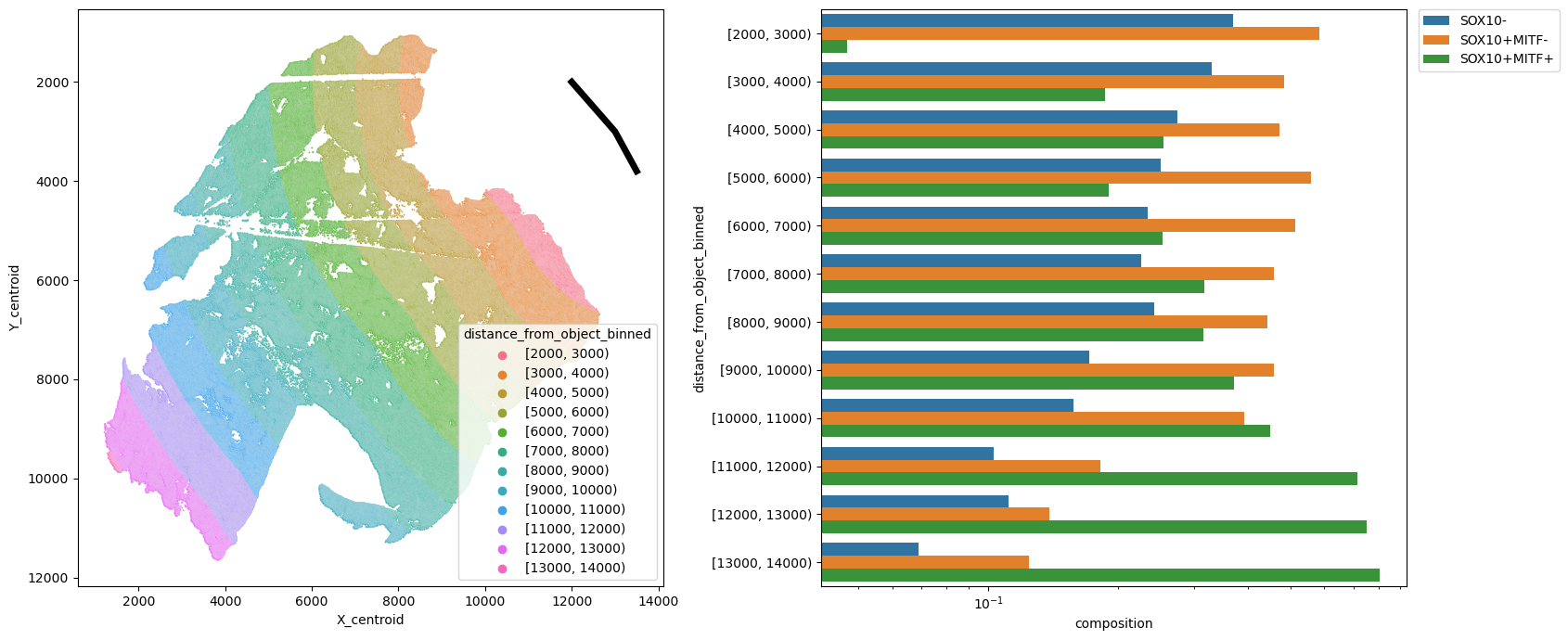
[27]:
metric_col = "distance_from_object_binned"
pheno = "pheno"
fig, [ax1, ax2] = plt.subplots(1, 2, figsize=(17, 7))
# plot manually drawn shape
ax1.plot(*shapely_obj.xy, color="k", linewidth=5)
sns.scatterplot(
data=adata.obs,
x="X_centroid",
y="Y_centroid",
hue=metric_col,
alpha=0.8,
s=2,
ax=ax1,
)
ax1.invert_yaxis()
ax1.legend(loc="lower right", title=metric_col)
df = spc.msmt.getRegionComposition(
adata, [metric_col, pheno], regions=["Tumor"]
).sort_values(by=metric_col, ascending=False)
sns.barplot(data=df, y=metric_col, x="composition", hue=pheno, log=True, ax=ax2)
ax2.legend(bbox_to_anchor=(1.02, 1), loc="upper left", borderaxespad=0)
plt.tight_layout()
plt.show()
/Users/ghwan/opt/anaconda3/envs/spatialcells/lib/python3.10/site-packages/seaborn/categorical.py:253: FutureWarning: The default of observed=False is deprecated and will be changed to True in a future version of pandas. Pass observed=False to retain current behavior or observed=True to adopt the future default and silence this warning.
grouped_vals = vals.groupby(grouper)
/Users/ghwan/opt/anaconda3/envs/spatialcells/lib/python3.10/site-packages/seaborn/categorical.py:253: FutureWarning: The default of observed=False is deprecated and will be changed to True in a future version of pandas. Pass observed=False to retain current behavior or observed=True to adopt the future default and silence this warning.
grouped_vals = vals.groupby(grouper)
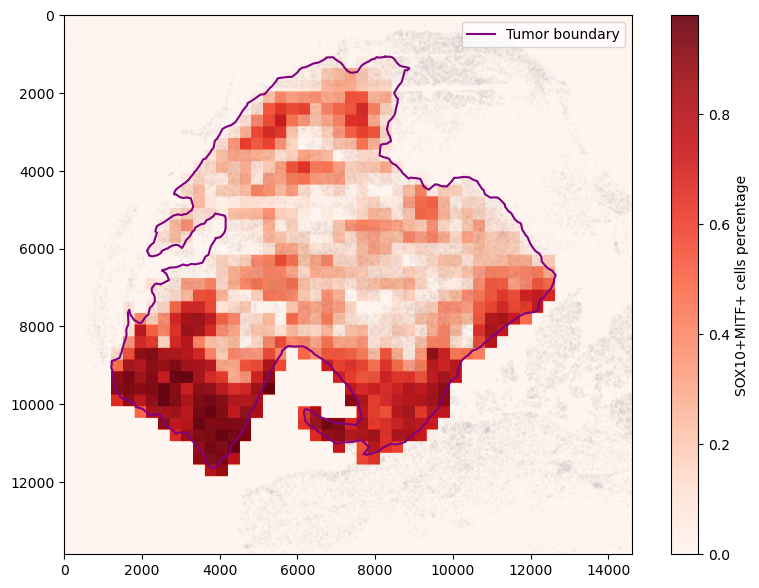
Sliding window analysis
We can also divide the tumor region into square windows and get the cell composition within each window. This can be matched with patch-level image analysis to provide additional features
[28]:
help(spc.msmt.getSlidingWindowsComposition)
Help on function getSlidingWindowsComposition in module spatialcells.measurements._getSlidingWindowsComposition:
getSlidingWindowsComposition(adata, window_size, step_size, phenotype_col, region_col='region', region_subset=None, min_cells=0)
Get Sliding window cell composition for cells in region subset.
:param adata: Anndata object
:param window_size: Size of the sliding window
:param step_size: Size of the step
:param phenotype_col: list of columns containing the cell type markers,
for cell type composition
:param region_col: Column containing the region information
:param region_subset: List of regions to consider. If None, consider all cells.
:param min_cells: Minimum number of cells in a window to consider it
:returns: A dataframe containing the cell type composition of the region in each window
[29]:
size = 300
adata1 = adata[adata.obs["SOX10_cellRingMask_positive"]]
mitf_df = spc.msmt.getSlidingWindowsComposition(
adata = adata1,
window_size = size,
step_size = size,
phenotype_col = "pheno",
region_subset=["Tumor"],
min_cells = 10
)
[30]:
mitf_df
[30]:
| pheno | cell_count | composition | X_start | Y_start | window_size | step_size | |
|---|---|---|---|---|---|---|---|
| 0 | SOX10+MITF- | 77 | 0.865169 | 1211 | 8562 | 300 | 300 |
| 1 | SOX10+MITF+ | 12 | 0.134831 | 1211 | 8562 | 300 | 300 |
| 0 | SOX10+MITF- | 215 | 0.520581 | 1211 | 8862 | 300 | 300 |
| 1 | SOX10+MITF+ | 198 | 0.479419 | 1211 | 8862 | 300 | 300 |
| 0 | SOX10+MITF+ | 643 | 0.883242 | 1211 | 9162 | 300 | 300 |
| ... | ... | ... | ... | ... | ... | ... | ... |
| 1 | SOX10+MITF+ | 169 | 0.288889 | 12311 | 6462 | 300 | 300 |
| 0 | SOX10+MITF- | 273 | 0.505556 | 12311 | 6762 | 300 | 300 |
| 1 | SOX10+MITF+ | 267 | 0.494444 | 12311 | 6762 | 300 | 300 |
| 0 | SOX10+MITF+ | 97 | 0.683099 | 12311 | 7062 | 300 | 300 |
| 1 | SOX10+MITF- | 45 | 0.316901 | 12311 | 7062 | 300 | 300 |
1605 rows × 7 columns
To plot non-overlapping windows, we can first create a numpy mask representing the composition for cell types of interest in each window.
[31]:
help(spc.msmt.get_comp_mask)
Help on function get_comp_mask in module spatialcells.measurements._getSlidingWindowsComposition:
get_comp_mask(df, pheno_col, pheno_vals, step_size)
Get a mask of the composition of the region in each window
:param df: A dataframe containing the cell type composition of pheno_vals in each window
:param pheno_col: Column containing the cell type information
:param pheno_vals: List of cell types to consider
:param step_size: Size of the step
:return: A np array mask of the composition of the region in each window
[32]:
fig, ax = plt.subplots(figsize=(10, 7))
mask = spc.msmt.get_comp_mask(mitf_df, "pheno", ["SOX10+MITF+"], size)
plt.imshow(mask, alpha=0.9, cmap="Reds", extent=(0, 1, 0, 1))
cbar = plt.colorbar()
cbar.ax.set_ylabel("SOX10+MITF+ cells percentage")
for region in sorted(set(adata.obs["region"])):
tmp = adata.obs[adata.obs.region == region].sample(frac=0.1)
ax.scatter(
*zip(*tmp[["X_centroid", "Y_centroid"]].to_numpy()),
s=1,
alpha=0.01,
color="grey"
)
# Bounds of points in selected communities
spc.plt.plotBoundary(pruned_boundary, color="purple", label="Tumor boundary")
ax.imshow(mask, alpha=1, cmap="Reds")
plt.legend(loc="upper right", markerscale=10)
plt.show()
[ ]: